12 atm is the new pressure of the air‑fuel mixture when A gaseous air‑fuel mixture in a sealed car engine cylinder has an initial volume of 600 ml at 1.0atm and final volume of 50 ml.
Step-by-step explanation:
Data given:
The air fuel mixture is assumed to be having ideal behaviour
initial volume of gaseous air fuel mixture V1 = 600 ml
initial pressure of gaseous air fuel mixture P1= 1 atm
final volume when piston is removed, V2= 50 ml
final pressure of the gaseous air mixture, P2 = ?
Applying the Boyle's Law,
P1V1 = P2V2
rearranging the equation:
P2 =
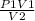
putting the value in the equation,
P2 =
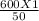
P2 = 12 atm
the pressure is increased to 12 atm when volume is reduced to 50 ml.