Answer:
1. 2.125 moles
2. 1,339.45 mL
3. 6.1079 grams
4. 5.7026 M
5. 0.1898 mol/L
Step-by-step explanation:
1. Molarity is defined as the number of moles of a substance per litre solution.
-Given the volume of the solution is 250 mL and 8.5 M of sulfuric acid, the moles is obtained as:
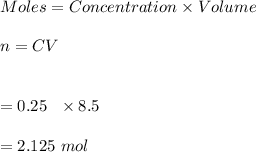
Hence, there are 2.125 moles of sulfuric acid in the solution.
2. Given 7.3 moles of sodium nitrate in a solution whose concentration is 5.45M.
#We apply the molarity formula to determine the solution's volume:

Hence, the volume of the solution is 1,339.45 mL
3. Given the mass of the solvent, methanol is 875 g and the molality of the solution is 0.430 m
#we apply the molality formula to calculate the mass of the solute :
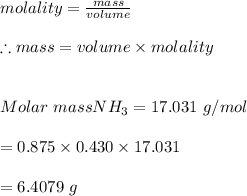
Hence, the mass of the solute is 6.4079 grams
4. Given the densities as
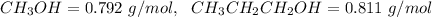
We apply the molality formula to find the molality of the solution as follows:
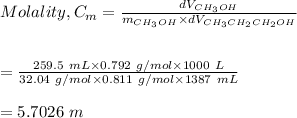
Hence, the molality of the solution is 5.7026 M
5. Molarity is defined as the number of moles of a solute in a 1000 ml of a solution:
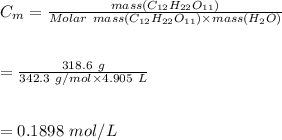
Hence, the molality of the solution is 0.1898 mol/L