Answer:
- Heating to remove water of crystallization
- Passing through a drying agent (deliquescent or hygroscopic substances)
Step-by-step explanation:
If solid/crystals, compound can be dried by applying heat to remove the water of crystallization.
Drying agents are also utile in removing moisture from compounds in a dessicator. Substances which absorb water from air can be used as drying agents for gases. For instance,
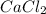 , CaO or silica gel is suitable for drying gases, depending on reactivity of the substance.
, CaO or silica gel is suitable for drying gases, depending on reactivity of the substance.