Answer: The final temperature of the water is
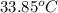 .
.
Step-by-step explanation:
We know that molar mass of
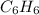 is 78 g/mol. And, the amount of heat produced when 2 mol of
is 78 g/mol. And, the amount of heat produced when 2 mol of
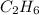 burns is 6542 KJ.
burns is 6542 KJ.
This means that,
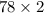 = 156 g of
= 156 g of
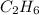 burns, heat produced is 6542 kJ.
burns, heat produced is 6542 kJ.
Therefore, heat produced (Q) by burning 7.3 g of
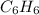 is as follows.
is as follows.
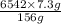
= 306.13 kJ
or, = 306130 J (as 1 KJ = 1000 J)
For water, mass is given as 5691 g and specific heat capacity of water is 4.186
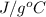 .
.
So, we will calculate the value of final temperature as follows.
Q =
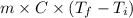
306130 J =
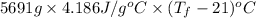
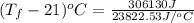
 = 12.85 + 21
= 12.85 + 21
=
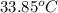
Thus, we can conclude that the final temperature of the water is
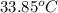 .
.