0.66 M is the accurate molarity of the new solution of volume of 1200 ml.
Step-by-step explanation:
Data given:
molarity of copper(II) sulphate, Mconc.= 2M
volume of 2M solution taken Vconc. = 400 ml
volume taken for dilution, Vdilute = 1200 ml
molarity of the diluted solution, Mdilute =?
We will use the formula for dilution as
Mconc Vconc = Mdilute x V dilute (conc is concentrated)
putting the values in the equation:
2 x 400 = Mdilute x 1200
Mdilute =
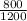
Mdilute = 0.66 M
When the solution is diluted to the volume of 1200 ml its molarity changes to 0.66 M.