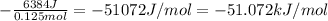Answer:
Change in enthalpy is -51.072 kJ/mol.
Step-by-step explanation:
Molar mass of NaOH = 39.997 g/mol
So, 4.98 g of NaOH =
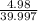 moles of NaOH = 0.125 moles of NaOH
moles of NaOH = 0.125 moles of NaOH
Total mass of solution = (4.98+52.79) g = 57.77 g
Heat consumed by solution =
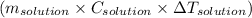 , where m is mass C is specific heat and
, where m is mass C is specific heat and
 is change in temperature.
is change in temperature.
So, heat consumed by solution =
![[57.77g* 4.186\frac{J}{g.^(0)\textrm{C}}* (50.1-23.7)^(0)\textrm{C}]](https://img.qammunity.org/2021/formulas/chemistry/high-school/z9rqs3eeb8h8yvtsv6e1ueikjqabnkt4x1.png)
= 6384 J
It is an exothermic process as temperature increases during dissolution. Hence change in enthalpy should be negative.
Change in enthalpy = -(heat consumed by solution)/(no. of moles of NaOH)
=