749.69 ml is the new volume if, with the pressure and amount of gas held constant the temperature is decreased to 253 K.
Step-by-step explanation:
Data given:
Initial volume of the neon gas V1 = 886 ml
initial pressure of the neon gas P1 = 752 torr or
initial temperature of the neon gas T1 = 299 K
Final volume =?
pressure constant so final pressure = initial pressure hence P2 = P1
fInal temperature of the neon gas T2 = 253 K
applying the Gay Lussac equation
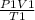 =
=
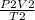
Pressure can be removed from the equation as it is constant, so we apply Charles' Law equation:
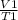 =
=
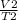
rearranging the above equation to know final volume:
V2=
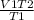
V2 =
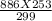
V2 = 749.69 ml
final volume is 749.69 ml