Answer:
1.7*10^{12}Ucm^-1
Step-by-step explanation:
The answer to this question is obtained by using the following formula:
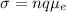
sigma: conductivity
q: charge of the electron = 1.61019*10^{-19}C
mu_e: electron mobility = 8104 m^2/Vs
n: free electron concentration = 1.31029/m^3
By replacing you get:
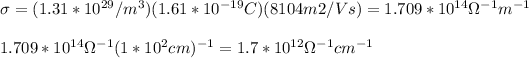
the result obained is not 5.9mU.cm. This is because temperature effects has not taken into account.