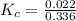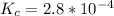Complete Question
The complete question is shown on the first uploaded image
Answer:
The equilibrium constant is
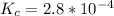
Step-by-step explanation:
From the question we are told that
The chemical reaction equation is
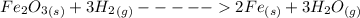
The voume of the misture is
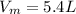
The molar mass of
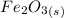 is a constant with value of
is a constant with value of
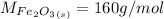
The molar mass of
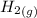 is a constant with value of
is a constant with value of
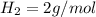
The molar mass of
 is a constant with value of
is a constant with value of
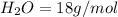
Generally the number of moles is mathematically given as
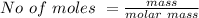
For
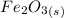
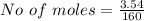
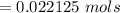
For

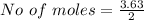
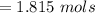
For

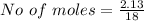
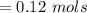
Generally the concentration of a compound is mathematicallyrepresented as
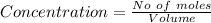
For
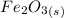
![Concentration[Fe_2 O_3] = (0.222125)/(5.4)](https://img.qammunity.org/2021/formulas/chemistry/college/bmv6rzsw3bcig34cucuo7gtcfajogpvbg6.png)
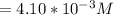
For

![Concentration[H_2] = (1.815)/(5.4)](https://img.qammunity.org/2021/formulas/chemistry/college/5fk2a94nflhqwqyxfkznxw4yafu6l7oo9l.png)
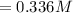
For

![Concentration [H_2O] = (0.12)/(5.4)](https://img.qammunity.org/2021/formulas/chemistry/college/hjdza1phie1d5xtwt9xc6vdt115qri7pav.png)
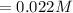
The equilibrium constant is mathematically represented as
![K_c = ([concentration \ of \ product])/([concentration \ of \ reactant ])](https://img.qammunity.org/2021/formulas/chemistry/college/kkfs6b13ls9l85ox1h11g0ol2tc1vrl1bk.png)
Considering
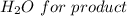
And
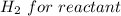
At equilibrium the