Answer:
The radius of curvature of the ion's orbit is 0.59 meters
Step-by-step explanation:
Given that,
Mass of the 24 Mg ion,
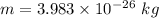
Potential difference, V = 3 kV
Magnetic field, B = 526 G
Charge on single ionized ion,
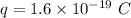
The radius of the the path traveled by the charge is circular. Its radius is given by :
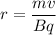
v is speed of particle.
v can be calculated using conservation of energy as :
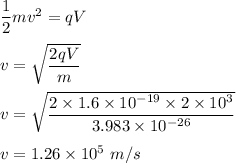
Radius,
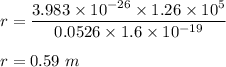
So, the radius of curvature of the ion's orbit is 0.59 meters.