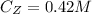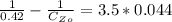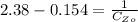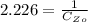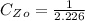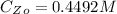Complete Question
The complete question is shown on the first uploaded image
Answer:
The concentration of
 that should used originally is
that should used originally is
Step-by-step explanation:
From the question we are told that
The necessary elementary step is
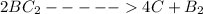
The time taken for sixth of 0.5 M of reactant to react
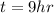
The time available is
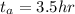
The desired concentration to remain
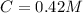
Let Z be the reactant , Y be the first product and X the second product
Generally the elementary rate law is mathematically as
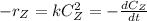
Where k is the rate constant ,
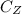 is the concentration of Z
is the concentration of Z
From the elementary rate law we see that the reaction is second order (This because the concentration of the reactant is raised to power 2 )
For second order reaction
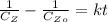
Where
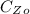 is the initial concentration of Z which a value of
is the initial concentration of Z which a value of
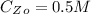
From the question we are told that it take 9 hours for the concentration of the reactant to become
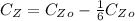
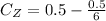
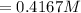
So
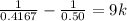
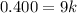
=>
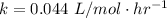
For