Answer:
a)
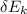 = 112.164 W
= 112.164 W
b)
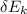 = 42.567 W
= 42.567 W
c) From what we've explained in part b; increase in temperature of the system is caused by rate of heat transfer . Therefore, not all heat is used to increase kinetic energy. Hence, since not all the heat is used to increase the kinetic energy . It is not valid and it is incorrect to say that rate of heat transfer is equal to the change in kinetic energy.
Step-by-step explanation:
The given data include:
Inlet diameter
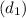 = 7 cm = 0.7 m
= 7 cm = 0.7 m
Inlet velocity
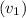 = 42 m/s
= 42 m/s
Inlet pressure
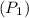 = 130 KPa
= 130 KPa
Inlet temoerature
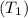 = 300°C = (300 + 273.15) = 573.15 K
= 300°C = (300 + 273.15) = 573.15 K
a) Assuming Ideal gas behaviour
Inlet Volumetric flowrate
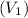 = Inlet velocity
= Inlet velocity
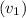 × area of the tube
× area of the tube
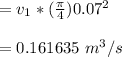
Using Ideal gas law at Inlet 1
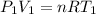
where ; n = molar flow rate of steam
making n the subject of the formula; we have:
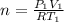
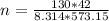
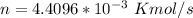
Moleular weight of air = 28.84 g/mol
The mass flow rate = molar flowrate × molecular weight of air
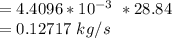
Finally: the kinetic energy at Inlet
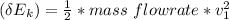
=
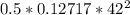
= 112.164 W
b) If the air is heated to 400°C;
Then temperature at 400°C = (400 + 273.15)K = 673.15 K
Thee pressure is also said to be constant ;
i.e
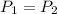 = 130 KPa
= 130 KPa
Therefore; the mass flow rate is also the same ; so as the molar flow rate:
Thus;
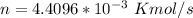
Using Ideal gas law at Inlet 2
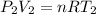
making
 the subject of the formula; we have:
the subject of the formula; we have:
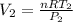
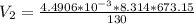
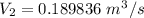
Assuming that the diameter is constant
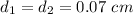
Now; the velocity at outlet =
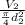
=
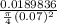
= 49.33 m/s
Change in kinetic energy
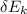 =
=
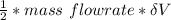
=
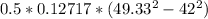
= 42.567 W
c).
From what we've explained in part b; increase in temperature of the system is caused by rate of heat transfer . Therefore, not all heat is used to increase kinetic energy. Hence, since not all the heat is used to increase the kinetic energy . It is not valid and it is incorrect to say that rate of heat transfer is equal to the change in kinetic energy.