Answer:
D) 18
Step-by-step explanation:
Hello,
In this case, by considering the given rate law:
![r=k[A]^2[B]](https://img.qammunity.org/2021/formulas/chemistry/high-school/k2sqcybmujl2t12z4u4tcw9mhkusuxfmwb.png)
We also take into account that the concentration of A is tripled and the concentration of B is doubled, therefore, we have:
![r=k[3*A]^2[2*B]](https://img.qammunity.org/2021/formulas/chemistry/high-school/ezbro786a3q37t0pph1bg6c8pk2w3ib9w8.png)
Which results in an increasing factor of:
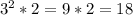
By considering only the tripling and the doubling, thus, the answer is D) 18.
Regards.