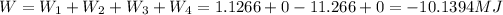Answer:
Step-by-step explanation:
For first isobaric process (expansion with constant pressure):
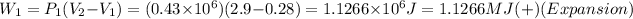
Now, for the first isochoric:
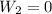 , and
, and
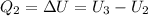
For the second isobaric process (compression with constant pressure):
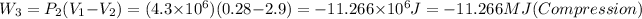
For the last isochoric:
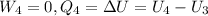
So, the total work per cycle: