Answer:
0.075M
Step-by-step explanation:
-The concentration of a solution is defined as the mass of the solute divided by the volume of the solution:
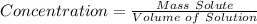
-We the solve the molarity problem as follows:
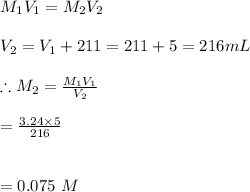
Hence, the concentration of the dilute solution is 0.075M