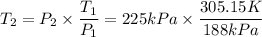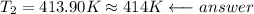Answer:
Step-by-step explanation:
The question is not completely clear because some missing parts or grammar (syntax) errors.
Interpreting it, the question is what is the Kelvin temperatue of the air in a tire, when the pressure in the tire has increased to 225 kPa, if the initial conditions of the air inside the tire were 188kPa of pressure and a temperature of 32ºC.
To solve this, you can assume constant volume and use the law of Gay-Lussac for ideal gases:
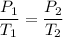
That equation works with absolute temperatures, i.e. Kelvin.
- 32ºC = 32 + 273.15 K = 305.15K
Then solve for T₂, substitute and compute: