Number of moles = 5.7 moles of oxygen.
Step-by-step explanation:
We have to convert number of molecules into number of moles by dividing the number of molecules by Avogadro's number.
Here number of molecules of oxygen given is 34.1 × 10²³ molecules.
Now we have to divide the number of molecules by Avogadro's number as,
Number of moles =
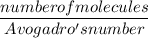
=
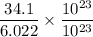
= 5.7 moles
So here molecules is converted into moles.