0.050 litres of the water will be required to make a 3.91 M solution with 0.196 moles in it.
Step-by-step explanation:
Data given:
moles of Cd
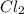 = 0.196 Moles
= 0.196 Moles
Molarity of the solution = 3.91 M
Volume in litres =?
molarity is calculated by the formula:
molarity =
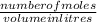
putting the values in the above formula and rearranging it for volume:
volume =
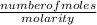
volume =
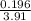
volume = 0.050 litres
0.050 litres of the water will be required to make a 3.91 M solution with 0.196 moles in it.
Molarity is the number of moles present in a given volume of solution which is given in litres. It is the measurement of the concentration of particular solute in a solution.