Answer:
74 or 74 kPa.
Step-by-step explanation:
Hello,
In this case, based on the initial information, it is seen that the oxygen and the carbon dioxide form the mixture at 160 kPa, thus, by isolating the oxygen, its pressure will be equal to its initial partial pressure because it gets isolated, hence, we compute its molar fraction as:
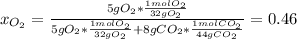
Therefore, its initial pressure turns out:
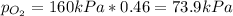
Such pressure will be the oxygen's pressure once it is isolated. Finally, considering the request, the answer will be just 74 (by rounding to the nearest integer and without units).
Best regards.