Answer:
The claim is false. (
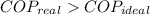 ).
).
Step-by-step explanation:
The real coefficient of performance of the food freezer is:
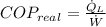
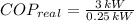
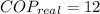
The ideal coefficient of performance, that is, when freezer has a reversible process, is:
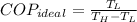
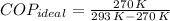
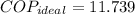
A real freezer has a coefficient of performance lesser than or equal to ideal coefficient of performance. Since supposed real coefficient of performance is greater than ideal coefficient of performance. The claim is proved to be false.