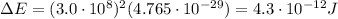Answer:
Step-by-step explanation:
The fusion reaction in this problem is
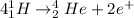
The total energy released in the fusion reaction is given by
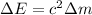
where
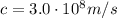 is the speed of light
is the speed of light
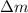 is the mass defect, which is the mass difference between the mass of the reactants and the mass of the products
is the mass defect, which is the mass difference between the mass of the reactants and the mass of the products
For this fusion reaction we have:
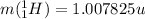 is the mass of one nucleus of hydrogen
is the mass of one nucleus of hydrogen
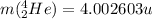 is the mass of one nucleus of helium
is the mass of one nucleus of helium
So the mass defect is:
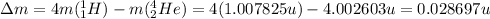
The conversion factor between atomic mass units and kilograms is
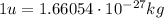
So the mass defect is
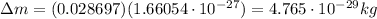
And so, the energy released is: