Answer: The volume of neon gas is, 56.7 L
Explanation :
To calculate the volume of gas we are using ideal gas equation:

where,
P = Pressure of neon gas = 0.37 atm
V = Volume of neon gas = ?
n = number of moles neon = 0.83 mole
R = Gas constant =
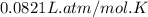
T = Temperature of neon gas =

Now put all the given values in above equation, we get:
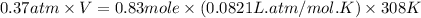
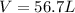
Therefore, the volume of neon gas is, 56.7 L