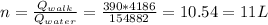Answer:
The number of liters of ice water is 11 L
Step-by-step explanation:
Given data:
normal body temperature = 37°C
temperature of the ice water = 0°C
Cwater = specific heat of water = 4186 J/kg °C
Suppose the person drinks 1 L of cold water, then, the mass is 1 kg
The heat is:
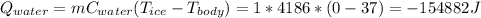
The sign (-) indicates the energy lost by the metabolic process. If the Qwalk is 390 kilocalories, then the number of liters of ice water is equal to: