Answer:
(1) Venus
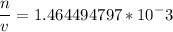
(2) Earth
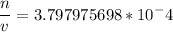
Step-by-step explanation:
Ideal Gas Equation which relates these quantities of thermodynamics is.
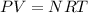 where P is pressure, V is volume , N is number of molecules, R is ideal Gas Constant and has value of 8.3144621J/Mol*K.
where P is pressure, V is volume , N is number of molecules, R is ideal Gas Constant and has value of 8.3144621J/Mol*K.
Rearranging the above equation, by first solving for N in terms of P, V, R and T and then dividing by V gives us.
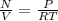
Now it is only matter of Plugging in numbers so for Venus planet we can plug in P = 9.0106Pa, T = 740K R is constant in every situation = 8.3144621J/mol K. and get (1) and similarly for Earth we will plug in P = 1.0105Pa , T = 320K and same R, and will get (2).