Answer: 1.3% many crosslinks as isoprene units,
Step-by-step explanation:
Given:
mass pf natural rubber= 200.0g
mass of sulphur = 4.8g
molar mass of sulphur =32g/mol
molar mass of isoprene = C5H8=( 12x5) +(1x8)= 68g/mol
Solution: we first find no of moles present in each using
no of moles =
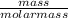
Isoprene: 200.0g x [1mole / 68g] = 2.94moles.
Sulfur: 4.8g x [1mole / 32g] x [1 mole crosslinks / 4 moles S] = 0.0375 moles crosslinks.
to find % crosslinked units, we have
0.0375 / 2.94 = 1.3% as many crosslinks as isoprene units,