Answer:
temperature T2 = 826.9°C
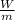 = -1142.7 kJ/kg
= -1142.7 kJ/kg
Step-by-step explanation:
given data
initial state temperature = 120°C
final state pressure p1 = 1 bar
pressure p2 = 100 bar
solution
we use here super heated water table A6 that is
specific internal energy u1 = 2537.3 kJ/kg
specific entropy s1 = 7.4668 kJ/kg.K
and
here specific entropy stage 1 = stage 2
so for specific entropy and pressure 100 bar
specific internal energy u2 = 3680 kJ/kg
and temperature T2 = 826.9°C
so here now we get specific work of steam is
ΔU = -W ...........1
m ( u1 - u2) = W
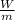 = u1 - u2
= u1 - u2
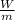 = 2537.3 - 3680
= 2537.3 - 3680
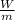 = -1142.7 kJ/kg
= -1142.7 kJ/kg