Answer:
1.24g
Step-by-step explanation:
Given parameters:
Mass of titanium (IV) chloride = 4.6g
Unknown:
Mass of oxygen = ?
Solution:
To solve this problem, we need to write the balance reaction equation first;
TiCl₂ + O₂ → TiO₂ + Cl₂
Now to find the mass of the oxygen gas,
Express the given mass of the known TiCl₂ in moles:
Number of moles of TiCl₂ =

Molar mass of TiC₂ = 47.9 + 2(35.5) = 118.9g/mol
Number of moles of TiCl₂ =
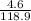 = 0.04mole
= 0.04mole
From the reaction equation;
1 mole of TiCl₂ reacted with 1 mole of oxygen gas
0.04 mole of TiCl₂ will react with 0.04 mole of oxygen gas
Mass of oxygen gas = number of moles x molar mass
molar mass of oxygen gas = 2(16) = 32g/mol
Mass of oxygen gas = 0.04 x 32 = 1.24g