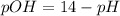Answer : The mathematical relationships gives the pOH of pure water at
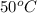 is,
is,
Explanation :
As we are given that the pH at
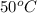 is, 6.6.
is, 6.6.
Now we have to determine the mathematical relationships gives the pOH of pure water at
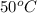 .
.
As we know that:
The ionization of water is:
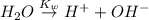
The expression for dissociation constant for water is:
![K_w=[H^+][OH^-]](https://img.qammunity.org/2021/formulas/chemistry/college/7jdjcuyzjoh0xkeaez8b0dl2066xe1tebt.png)
taking logarithm on both side, we get:
![\log K_w=\log [H^+]+\log [OH^-]](https://img.qammunity.org/2021/formulas/chemistry/college/p1mx5gnewbtl47bg5x4pqxydjm8b09p2sk.png)
Taking negative sign on both side, we get:
![-\log K_w=-\log [H^+]+(-\log [OH^-])](https://img.qammunity.org/2021/formulas/chemistry/college/3xasxodkic2gnqh31hj9loyl5jr864xvup.png)
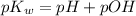
As we know that the value of
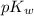 is 14 at 25-50°C.
is 14 at 25-50°C.
So,
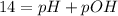
or,
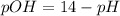
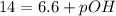
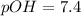
Therefore, the mathematical relationships gives the pOH of pure water at
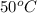 is,
is,