Answer:
Source 2.
Step-by-step explanation:
The efficiency of the ideal reversible heat engine is given by the Carnot's power cycle:
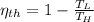
Where:
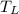 - Temperature of the cold reservoir, in K.
- Temperature of the cold reservoir, in K.
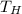 - Temperature of the hot reservoir, in K.
- Temperature of the hot reservoir, in K.
The thermal efficiencies are, respectively:
Source 1

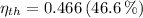
Source 2

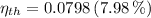
The power produced by each device is presented below:
Source 1
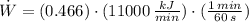
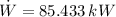
Source 2
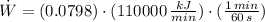
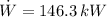
The source 2 produces the largest amount of power.