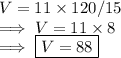Answer:
88 in³
Explanation:
we are given that the volume V of a given mass of gas varies directly as the temperature T and inversely as the pressure P.
Condition-1:
The volume V of a given mass of gas varies directly as the temperature T , thus
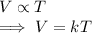
Condition-1:
The volume V of a given mass of gas varies inversely as the pressure P, therefore,
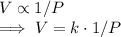
combining 1 and 2 yields
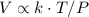
Case-1:
- V = 341.0 in³
- T = 310°
- P=10 lb/in²
To find:
Finding the constant of proportionality
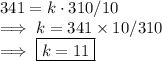
Case-1:
To find:
when:
Finding the volume: