Answer:
The total time (in seconds) for the transformation to reach 95% completion is 516.783 secs
Step-by-step explanation:
Given Data:
n=1.5
Time to reach 30% completion=125 Secs
Required:
Total Time for the transformation to reach 95% completion=?
Solution:
Avrami Equation:
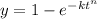
where:
k is time constant
t is the time
y is the remaining amount of reaction.
First Calculate K:
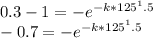
Taking "ln"on both sides
ln(0.7)=-k*(125^1.5)
k=0.000255
At 95 % completion:
Avrami Equation Can be rearranged as:
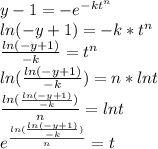
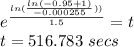
The total time (in seconds) for the transformation to reach 95% completion is 516.783 secs