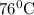Answer:
Water will boil at
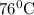 .
.
Step-by-step explanation:
According to clausius-clapeyron equation for liquid-vapor equilibrium:
![ln((P_(2))/(P_(1)))=(-\Delta H_(vap)^(0))/(R)[(1)/(T_(2))-(1)/(T_(1))]](https://img.qammunity.org/2021/formulas/chemistry/high-school/3ckodjxtbncppkkedoid9n6398tccdarxf.png)
where,
 and
and
 are vapor pressures of liquid at
are vapor pressures of liquid at
 (in kelvin) and
(in kelvin) and
 (in kelvin) temperatures respectively.
(in kelvin) temperatures respectively.
Here,
 = 760.0 mm Hg,
= 760.0 mm Hg,
 = 373 K,
= 373 K,
 = 314.0 mm Hg
= 314.0 mm Hg
Plug-in all the given values in the above equation:
![ln((314.0)/(760.0))=(-40.7* 10^(3)(J)/(mol))/(8.314(J)/(mol.K))* [(1)/(T_(2))-(1)/(373K)]](https://img.qammunity.org/2021/formulas/chemistry/high-school/lt7sdxggmlmaqyg5nerorypmolnobo0akg.png)
or,
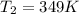
So,
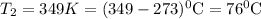
Hence, at base camp, water will boil at