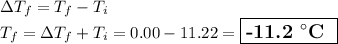Answer:
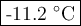
Step-by-step explanation:
The formula for the change in freezing point ΔT_f caused by an electrolyte is

1. Calculate i
Na₂SO₄(aq) ⟶ 2Na⁺(aq) + SO₄²⁻(aq)
1 mol Na₂SO₄ gives 3 mol ions.
i = 3
2. Calculate the freezing point
(a) Change in freezing point
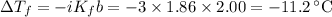
(b) Freezing point