The answer for the following problem is mentioned below.
- Therefore the final volume of the gas is 52.7 ml.
Step-by-step explanation:
Given:
Initial pressure (
 ) = 290 kPa
) = 290 kPa
Final pressure (
 ) = 104 kPa
) = 104 kPa
Initial volume (
 ) = 18.9 ml
) = 18.9 ml
To find:
Final volume (
 )
)
We know;
From the ideal gas equation;
P × V = n × R × T
where;
P represents the pressure of the gas
V represents the volume of gas
n represents the no of the moles
R represents the universal gas constant
T represents the temperature of the gas
So;
P × V = constant
P ∝
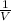
From the above equation;
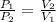
 represents the initial pressure of the gas
represents the initial pressure of the gas
 represents the final pressure of the gas
represents the final pressure of the gas
 represents the initial volume of the gas
represents the initial volume of the gas
 represents the final volume of the gas
represents the final volume of the gas
Substituting the values of the above equation;
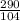 =
=
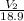
 = 52.7 ml
= 52.7 ml
Therefore the final volume of the gas is 52.7 ml.