The statement is false as 85675 grams of nitrogen dioxide is produced instead of 20 grams.
Step-by-step explanation:
Balance equation for the reaction:
2NO +
 ⇒ 2N
⇒ 2N

Data given:
mass of nitrogen produced = 20 gram
mass of oxygen = 29.8 litres or 29800 grams
0xygen is the limiting reagent so,
number of moles of oxygen: (atomic mass of 1 mole 32 grams/mole
number of moles =

putting the values in the equation:
=
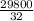
= 931.5 moles
1 mole of oxygen reacts to give 2 moles of NO2
931.5 moles of oxygen will give x moles
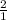 =
=
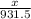
1862.5 moles of NO2 produced
in grams
mass = number of moles x atomic mass
mass = 1862.5 x 46.00
= 85675 grams
The statement is false as 85675 grams of nitrogen dioxide is produced instead of 20 grams as said in question.