Answer:
9.4g
Step-by-step explanation:
Given parameters:
Volume of hydrogen gas at STP = 15L
Unknown:
Mass of solid lithium = ?
Solution:
The reaction equation is shown below;
2Li + 2H₂O → 2LiOH + H₂
To solve this problem, lets work from the known to the unknown. The known here is hydrogen gas;
At STP;
number of moles =
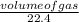 =
=
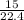 = 0.67mole
= 0.67mole
From the balanced reaction equation;
1 mole of hydrogen gas is produced from 2 moles of Li;
0.67 moles of hydrogen will be produced from 0.67 x 2 = 1.34moles
Mass of solid lithium = number of moles x molar mass
= 1.34 x 7
=9.4g