Answer:
- Percent deviation = 1.21%
Step-by-step explanation:
The statement gives some properties of a sample of carbon dioxide collected during the experiment of a combustion reaction and asks to determine the percent deviation of the experiment.
Then, what you must calculate is the percent error on the mass.
That is:
% error = |measured mass - theoretical mass| / (theoretical mass) × 100
The measured mass was 4.157g
The theoretical mass can be calculated from the data of pressure, volume, and temperature, using the ideal gas equation:
- p = 856.3 torr × 1 atm/ 760.0 torr = 1.1267 atm
- V = 2.498 liter
- T = 85.6 + 273.15 = 358.75K
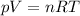
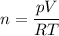
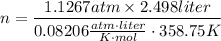
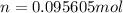
Now use the molar mass of CO₂ to calculate the theoretical mass:
- mass = number of moles × molar mass
- mass = 0.095605mol × 44.01g/mol = 4.20758g ≈ 4.208g
Finally, you can calculate the percent deviation or error:
- % error = |4.157g - 4.208g| / 4.208g × 100 = 1.21%