Answer:
(a)The solution is basic in nature.
(b)The solution is neutral in nature.
(c)The solution is acidic in nature.
Step-by-step explanation:
pH value:
- The value of pH Neutral solution is equal to 7.
- The value of pH of Acidic solution is less than 7.
- The value of pH of basic solution is grater than 7.
The concentration of H⁺ ion's (pH) of a solution is the magnitude of negative sign of logarithm value of [H₃O⁺}, is called pH of that solution.
That is
![pH=-log[H_3O^+]](https://img.qammunity.org/2021/formulas/chemistry/high-school/ii42rdd0lrihbaohdoeleatdk40jexti8r.png)
We know that,
![[H_3O^+][OH^-]=K_w=10^(-14)](https://img.qammunity.org/2021/formulas/chemistry/high-school/v13n769qj5ob71bw2krlr2sqnlp05acvi7.png) [ at 25°C]
[ at 25°C]
Taking log both sides
![log[H_3O^+]+log[OH^-]=logK_w=log 10^(-14)](https://img.qammunity.org/2021/formulas/chemistry/high-school/vym3ufg3p8xqe70sdfrfs97mch7jtth46d.png)
![\Rightarrow - log[H_3O^+]-log[OH^-]=-logK_w=-log 10^(-14)](https://img.qammunity.org/2021/formulas/chemistry/high-school/dzpeafwd0bnbzv0f8mcf9o6uu9mek2ra1a.png)
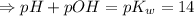
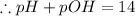
(a)
![[OH^-]=3.8*10^(-2)M](https://img.qammunity.org/2021/formulas/chemistry/high-school/291np06u91i139qv5wu75gq8wnvkhfdz45.png)
![- log[H_3O^+]-log[OH^-]=14}](https://img.qammunity.org/2021/formulas/chemistry/high-school/fa0vzyzn24gfthsuo4pqsf0hq0yyiwyyrw.png)
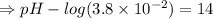
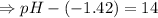
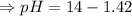
∴pH= 12.58
since pH>7
The solution is basic in nature.
(b)
![[OH^-]=1.0*10^(-7)M](https://img.qammunity.org/2021/formulas/chemistry/high-school/qu05fugy6ijgspzfrkudnf37epeja0l3i5.png)
![- log[H_3O^+]-log[OH^-]=14}](https://img.qammunity.org/2021/formulas/chemistry/high-school/fa0vzyzn24gfthsuo4pqsf0hq0yyiwyyrw.png)
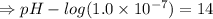
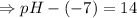
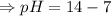
∴pH= 7
The solution is neutral in nature.
(c)
![[OH^-]=5.5*10^(-10)M](https://img.qammunity.org/2021/formulas/chemistry/high-school/pr1slungrs2sg2xkr0s9va96w7yt4e9b8g.png)
![- log[H_3O^+]-log[OH^-]=14}](https://img.qammunity.org/2021/formulas/chemistry/high-school/fa0vzyzn24gfthsuo4pqsf0hq0yyiwyyrw.png)
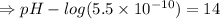
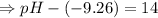
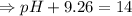
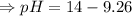
∴pH= 4.74
Since pH<7
The solution is acidic in nature.