Answer: The pH of the buffer after addition of given amount of HI is 4.76
Step-by-step explanation:
We are given:
Initial moles of propionic acid = 0.17 moles
Initial moles of sodium propionate = 0.14 moles
Moles of HI added = 0.01 moles
The chemical equation for the reaction of HI and sodium propionate follows:
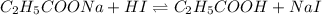
Initial: 0.14 0.01 0.17
At eqllm: 0.13 - 0.18
Total volume of the container = 1.20 L
To calculate the pH of acidic buffer, we use the equation given by Henderson Hasselbalch:
![pH=pK_a+\log(([salt])/([acid]))](https://img.qammunity.org/2021/formulas/biology/college/6usxe642bp3w274zbcv30her0kcessu95f.png)
![pH=pK_a+\log(([C_2H_5COONa])/([C_2H_5COOH]))](https://img.qammunity.org/2021/formulas/chemistry/college/19t1khox92p8ocvda1fs5allfphqaq9afx.png)
We are given:
 = negative logarithm of acid dissociation constant of propionic acid = 4.87
= negative logarithm of acid dissociation constant of propionic acid = 4.87
![[C_2H_5COONa]=(0.14)/(1.20)](https://img.qammunity.org/2021/formulas/chemistry/college/ko4me667annyi466fhnmgud66puk05z2br.png)
![[C_2H_5COOH]=(0.18)/(1.20)](https://img.qammunity.org/2021/formulas/chemistry/college/3y9dyxqow7zg2xcfvxf1qynh82yqe3xn75.png)
pH = ?
Putting values in above equation, we get:
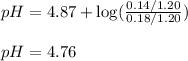
Hence, the pH of the buffer after addition of given amount of HI is 4.76