Answer:
The new temperature of the balloon is 27.12⁰C
Step-by-step explanation:
Given;
initial temperature of helium gas in balloon, T₁ = 24⁰ C
initial volume of the gas, V₁ = 0.0042 m³
pressure in the balloon, P = 101.3 kPa
The sun caused the balloon to expand by 13 % of the original volume; this implies that the original volume increased by 13%.
The new temperature, T₂ is calculated using general gas law;
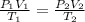
Since the pressure in the balloon is always equal, then P₁ = P₂
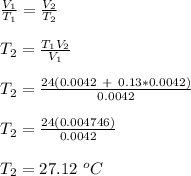
Therefore, the new temperature of the balloon is 27.12⁰C