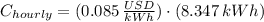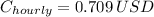Answer:
70.9 cents.
Step-by-step explanation:
Let assume that water at inlet is a saturated liquid. The process within pump is modelled after the First Law of Thermodynamics:
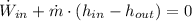
The power consumed by the pump is:
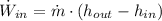
The isentropic effciency of the pump is:
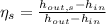
Where,
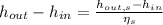
The power equation is modified into the following form:
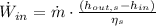
Specific enthalpies and entropies at inlet and outlet are obtained from steam tables and included below:
State 1 (Saturated Liquid)
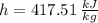
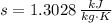
State 2s (Subcooled Liquid)
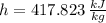
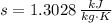
The power consume by the pump is:
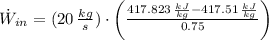
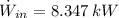
The hourly energy consumption is:
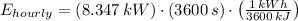
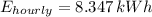
The hourly cost is: