Answer:
Non steady state process
Because D ∝ 1/t
Step-by-step explanation:
Here we have

Where:
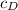 = 0.0025 % by weight of N
= 0.0025 % by weight of N
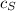 = 0.45 % by weight of N
= 0.45 % by weight of N
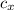 = 0.12 % by weight of N
= 0.12 % by weight of N
Therefore,
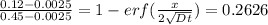
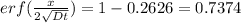
From the inverse erf function we have
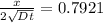
Since x = 0.45 mm, we have
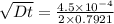 and
and
Dt = 8.069×10⁻⁸ m²
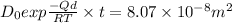
For steady state
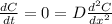
Since Dt = 8.069×10⁻⁸ m² = Constant, then as the time increases the diffusion rate decreases hence the process is not steady state.
D is inversely proportional to t.