Answer:
Therefore 21.09 gram of the element remains after 17 years.
Explanation:
The decay rate is proportional to the number of nuclei.
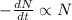
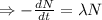
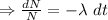
Integrating both sides
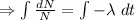
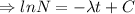
Initially N=
 , when t=0
, when t=0

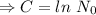
The equation becomes
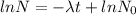
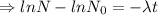
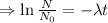
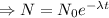
N= Remaining mass after t time
 = initial mass of the sample
= initial mass of the sample
 = decay constant
= decay constant
Given that, every 12 years the mass of a sample of a the element is one third of the initial mass.
Here,
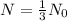 , t= 12 years
, t= 12 years
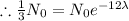
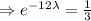
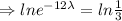
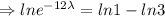 [
[
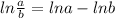 ]
]
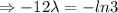 [
[
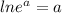 and
and
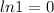 ]
]
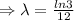
The equation becomes
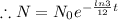
Given that, the initial amount
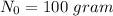 and t =17
and t =17
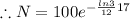
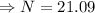 gram
gram
Therefore 21.09 gram of the element remains after 17 years.