Answer:
The temperature is 2584.5 K
Step-by-step explanation:
Given:
Activation energy
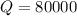
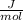
Preexponential
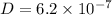
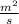
Diffusion flux
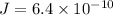
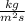
Thickness of plate
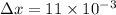 m
m
Concentration of carbon at two faces

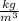
From the formula of temperature in terms of diffusion flux,
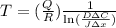
Where
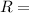 8.314
8.314
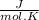 ( gas constant )
( gas constant )
Put the values and find the temperature,
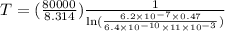
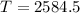 K
K
Therefore, the temperature is 2584.5 K