Answer:
0.085 moles of oxygen needed to produce 0.085 moles of water.
Step-by-step explanation:
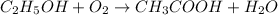
Moles of water required to produce = 0.085 mol
According to reaction, 1 mole of water is obtained from 1 mole of oxygen gas, then 0.085 mole of water will be obtained:
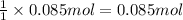 of oxygen gas
of oxygen gas
0.085 moles of oxygen needed to produce 0.085 moles of water.