Answer:
2.78x10⁻³ m
Step-by-step explanation:
Nitrogen diffusion in the steel can be expressed using the following equation:
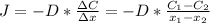
Where:
J: is the diffusion flux = 1.0x10⁻⁷ kgm⁻²s⁻¹
D: is the diffusion coefficient = 1.85x10⁻¹⁰ m²/s
C₁: is the concentration of nitrogen in the steel at the surface = 2 kg/m³
C₂: is the concentration of nitrogen at the distance x₂ = 0.5 kg/m³
x₁: is the distance at the surface = 0
x₂: is the distance to find
Hence, we have:
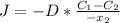 (1)
(1)
Solving equation (1) for x₂:
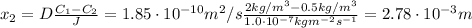
Therefore, the nitrogen will enter 2.78x10⁻³ m into the sheet.
I hope it helps you!