Answer:
Initial mass = 5.91 kg
Final mass = 16.8 kg
Heat transfer Q = - 625.9 KJ
Step-by-step explanation:
Given data
Tank volume V = 1

Entering outside air temperature
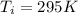
Entering outside air pressure
 = 15 bar
= 15 bar
Initial tank pressure
 = 5 bar
= 5 bar
Initial tank temperature
 = 295 K
= 295 K
Final pressure
 = 15 bar
= 15 bar
Final temperature
 = 310 K
= 310 K
We know that
P V = m R T
(a). Initial mass is given by
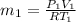
Put all the values in given equation
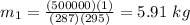
(b). Final mass is given by
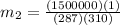
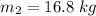
This is the final volume of the tank.
Δ U = Δ q +
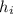 Δ
Δ
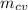
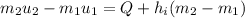
Specific internal energy at initial temperature & pressure
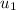 = 210.5
= 210.5
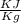
Specific internal energy at final temperature & pressure
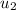 = 221.25
= 221.25
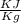
Specific enthalpy is
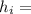 295.17
295.17
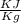
Q = ( 16.8 × 210.48 - 5.91 × 210.49 )- 295.17 ( 16.8 - 5.91 )
Q = 2292.23 - 3214.4
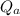 = - 741.4 KJ
= - 741.4 KJ
The heat transfer for the tank is given by
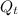 = m C
= m C
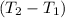
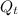 = 20 × 0.385 × ( 310-295 )
= 20 × 0.385 × ( 310-295 )
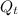 = + 115.5 KJ
= + 115.5 KJ
Total heat transfer Q =
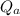 +
+
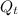
Q = - 741.4 + 115.5
Q = - 625.9 KJ
This is the heat transfer to the surrounding from the tank.