Only option a and the option b follows the first law of thermodynamics.
Step-by-step explanation:
As we know that the first law of thermodynamics tells the conservation of energy, that is why it is also called quantitative law.
From the first law of thermodynamics
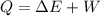
a. Given that
W= 50 KJ,Q = 170KJ ,ΔE = 120 KJ
Yes, this follows the first law of thermodynamics.
b. Given that
W= 100 Btu,Q= -110 Btu ,ΔE= -210 Btu
This also satisfy the first law of thermodynamics.
c. Given that
W= 250 KJ,Q= -110 KJ ,ΔE=-100 KJ
Yes this does not follow the first law of thermodynamics.
d. Given that
Yes, this does not follow the first law of thermodynamics.