Answer:
final temperature T = 24.84ºC
Step-by-step explanation:
given data
copper volume = 1 L
temperature t1 = 500ºC
oil volume = 200 L
temperature t2 = 20ºC
solution
Density of copper
 cu = 8940 Kg/m³
cu = 8940 Kg/m³
Density of light oil
 oil = 889 Kg/m³
oil = 889 Kg/m³
Specific heat capacity of copper Cv = 0.384 KJ/Kg.K
Specific heat capacity of light oil Cv = 1.880 KJ/kg.K
so fist we get here mass of oil and copper that is
mass = density × volume ................1
mass of copper = 8940 × 1 ×
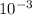 = 8.94 kg
= 8.94 kg
mass of oil = 889 × 200 ×
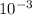 = 177.8 kg
= 177.8 kg
so we apply here now energy balance equation that is
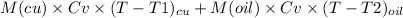 = 0
= 0
put here value and we get T2
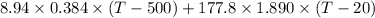 = 0
= 0
solve it we get
T = 24.84ºC