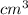The density of the compound is 4.64 g/
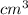 .
.
Step-by-step explanation:
Sodium chloride crystallize as FCC face centered cubic structure. It consist of four atoms of NaCl. So the unknown compound which crytallizes like NaCl wil have volume as:
radius in fcc is calculated as
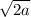 = 4r
= 4r
putting the value where a is length of the edge.
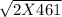
= 651.9 = 4r so r= 162.98 pm
volume is calculated by the formula:
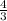 π
π
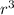
putting the values,
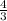 x 3.14 x (
x 3.14 x (
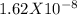 )^3
)^3
= 4.25X 10 ^-24
1.7 X
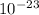
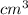 = volume of the fcc cell.
= volume of the fcc cell.
mass of the substance = 4 x 120 x 1.6605 x 10^(-24) g/u) (as it contains four atoms)
= 7.9x
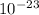
density =

putting the values
density =
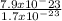
density = 4.64 g/